Colombia Orthopedic Devices Market (2025-2031) Outlook | Industry, Growth, Trends, Forecast, Analysis, Value, Size, Revenue, Share & Companies
| Product Code: ETC368104 | Publication Date: Aug 2022 | Updated Date: Jul 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
Colombia Orthopedic Devices Market Size Growth Rate
The Colombia Orthopedic Devices Market is projected to witness mixed growth rate patterns during 2025 to 2029. Commencing at 6.72% in 2025, growth builds up to 6.86% by 2029.
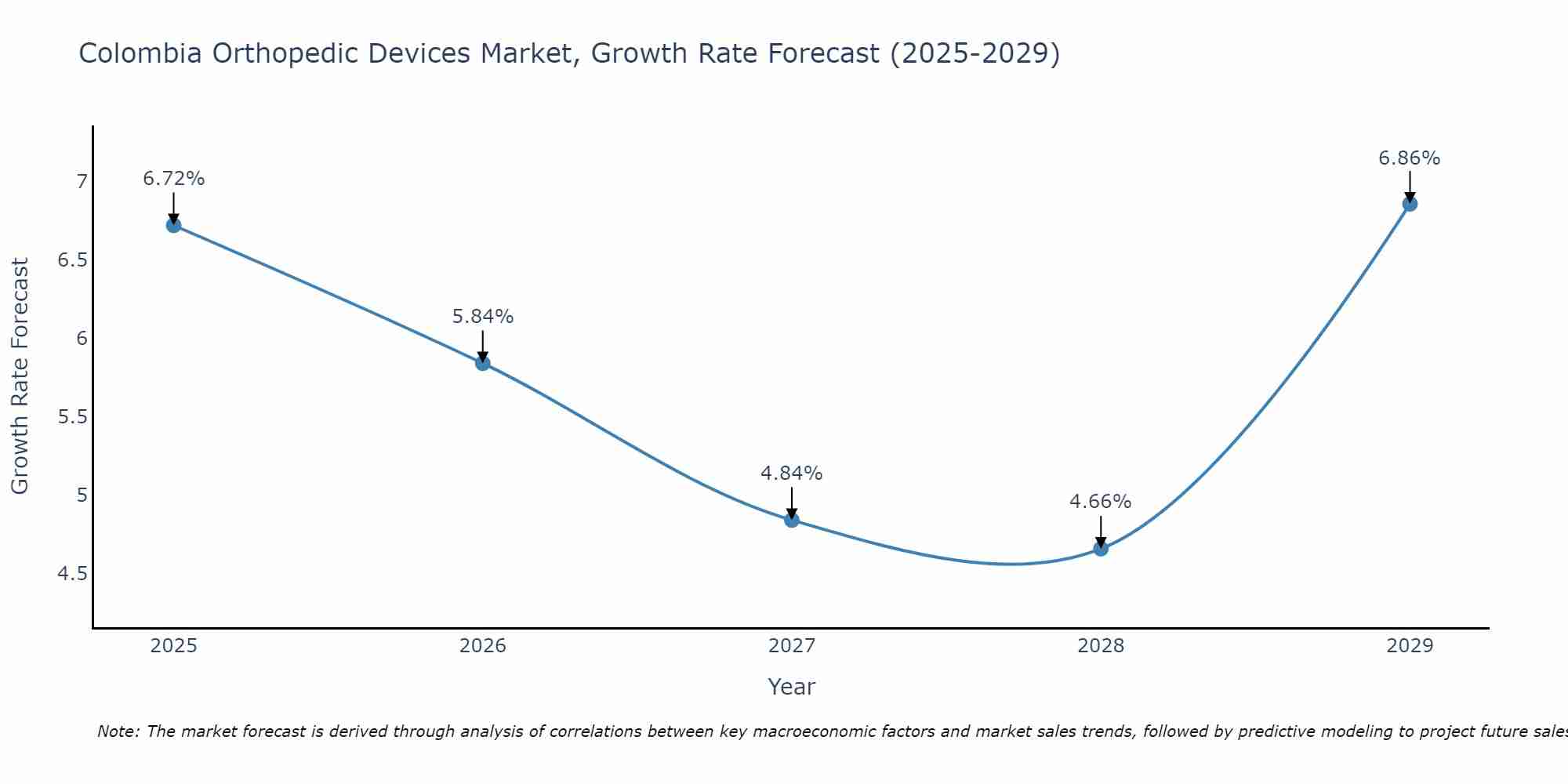
Colombia Orthopedic Devices Market Synopsis
The Colombia orthopedic devices market is witnessing significant growth due to factors such as an increasing aging population, rising prevalence of orthopedic disorders, and improving healthcare infrastructure. The market is characterized by a wide range of products including orthopedic implants, prosthetics, orthobiologics, and orthopedic accessories. Key players in the market are investing in research and development to introduce technologically advanced products, focusing on innovation and customization to meet the diverse patient needs. Government initiatives to improve access to healthcare services and increasing awareness about orthopedic disorders are further driving market growth. With a growing demand for minimally invasive procedures and the adoption of advanced surgical techniques, the Colombia orthopedic devices market is poised for continued expansion in the coming years.
Colombia Orthopedic Devices Market Trends
As of the latest data available, the Colombia Orthopedic Devices Market is experiencing significant growth driven by factors such as an increasing aging population, rising prevalence of orthopedic disorders, and advancements in technology. There is a growing demand for minimally invasive orthopedic procedures and implants, leading to a surge in the adoption of innovative orthopedic devices. Companies are focusing on developing advanced materials and technologies to improve the durability and performance of orthopedic implants. Additionally, the market is witnessing a trend towards personalized orthopedic solutions to meet the specific needs of patients. The market is also characterized by competition among key players, leading to product innovations and strategic collaborations to expand their market presence in Colombia. Overall, the Colombia Orthopedic Devices Market is poised for further growth in the coming years.
Colombia Orthopedic Devices Market Challenges
In the Colombia Orthopedic Devices Market, challenges are primarily related to regulatory issues, reimbursement policies, and market access. The regulatory environment in Colombia can be complex and difficult to navigate, leading to delays in product approvals and market entry. Reimbursement policies may vary and impact the affordability of orthopedic devices for patients, affecting market demand. Additionally, achieving market access and building relationships with healthcare providers can be challenging due to the presence of well-established local competitors and a preference for traditional treatment methods. Moreover, economic instability and fluctuations in currency exchange rates can also pose challenges for international orthopedic device manufacturers operating in Colombia. Overall, overcoming these hurdles requires a deep understanding of the local market dynamics and proactive strategies to address regulatory and reimbursement challenges.
Colombia Orthopedic Devices Market Investment Opportunities
The Colombia Orthopedic Devices Market offers promising investment opportunities due to the increasing demand for orthopedic implants and devices driven by the country`s aging population and rising incidences of orthopedic disorders. Key growth areas include joint reconstruction implants, spinal implants, trauma fixation devices, and orthobiologics. The market is also benefiting from technological advancements, improving healthcare infrastructure, and a growing emphasis on improving patient outcomes. Investors can explore partnerships with local distributors, acquisitions of Colombian orthopedic device manufacturers, or strategic collaborations with healthcare providers to capitalize on the market`s potential. Additionally, focusing on innovative solutions that cater to the specific needs of the Colombian population, such as affordable and high-quality orthopedic devices, can lead to long-term success in this evolving market.
Jordan Agar Market Government Policies
The Colombian government has implemented a series of policies to regulate the Orthopedic Devices Market. These policies include the requirement for orthopedic devices to be registered with the National Institute of Food and Drug Surveillance (INVIMA) to ensure safety and quality standards are met. Additionally, there are regulations in place to control pricing and reimbursement schemes to make orthopedic devices more accessible to the population. The government also promotes research and development in the orthopedic sector through tax incentives and grants to encourage innovation and technological advancements. Overall, these policies aim to safeguard public health, improve access to orthopedic devices, and foster growth in the Colombian orthopedic market.
Colombia Orthopedic Devices Market Future Outlook
The Colombia Orthopedic Devices Market is expected to witness steady growth in the coming years due to factors such as an increasing aging population, rising prevalence of orthopedic disorders, and advancements in technology leading to the development of innovative orthopedic devices. The market is also likely to benefit from the growing healthcare infrastructure and improving access to healthcare services in Colombia. Additionally, the rise in sports-related injuries and road accidents further contribute to the demand for orthopedic devices in the country. However, pricing pressures and reimbursement challenges may pose some constraints to market growth. Overall, the Colombia Orthopedic Devices Market is projected to expand as the healthcare sector continues to evolve and cater to the orthopedic needs of the population.
Key Highlights of the Report:
- Colombia Orthopedic Devices Market Outlook
- Market Size of Colombia Orthopedic Devices Market, 2024
- Forecast of Colombia Orthopedic Devices Market, 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Revenues & Volume for the Period 2021 - 2031
- Colombia Orthopedic Devices Market Trend Evolution
- Colombia Orthopedic Devices Market Drivers and Challenges
- Colombia Orthopedic Devices Price Trends
- Colombia Orthopedic Devices Porter's Five Forces
- Colombia Orthopedic Devices Industry Life Cycle
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Hip Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Knee Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Spine Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Craniomaxillofacial Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Dental Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Sports Injuries, Extremities And Trauma (Set) Orthopedic Devices for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Product for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Drill Guide for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Guide Tubes for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Implant Holder for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Custom Clamps for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Distracters for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Screw Drivers for the Period 2021 - 2031
- Historical Data and Forecast of Colombia Orthopedic Devices Market Revenues & Volume By Accessories for the Period 2021 - 2031
- Colombia Orthopedic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By Product
- Colombia Orthopedic Devices Top Companies Market Share
- Colombia Orthopedic Devices Competitive Benchmarking By Technical and Operational Parameters
- Colombia Orthopedic Devices Company Profiles
- Colombia Orthopedic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- Portugal Electronic Document Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- France Electronic Document Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Portugal Occupational Health & Safety Services Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Netherlands Occupational Health and Safety Services Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Belgium and Luxembourg Facility Management Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Russia Women Intimate Apparel Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Africa Chocolate Market (2025-2031) | Size, Share, Trends, Growth, Revenue, Analysis, Forecast, industry & Outlook
- Global Hydroxychloroquine And Chloroquine Market (2025-2031) | Industry, Trends, Size, Outlook, Growth, Value, Companies, Revenue, Analysis, Share, Forecast
- Saudi Arabia Plant Maintenance Market (2025-2031) | Industry, Size, Growth, Revenue, Value, Companies, Forecast, Analysis, Share & Trends
- Taiwan Electric Truck Market (2025-2031) | Outlook, Industry, Revenue, Size, Forecast, Growth, Analysis, Share, Companies, Value & Trends
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













