United States (US) Ophthalmic Devices Market Outlook | Trends, Industry, Analysis, Size, COVID-19 IMPACT, Companies, Share, Growth, Revenue, Forecast & Value
| Product Code: ETC367861 | Publication Date: Aug 2022 | Updated Date: Jul 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Summon Dutta | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
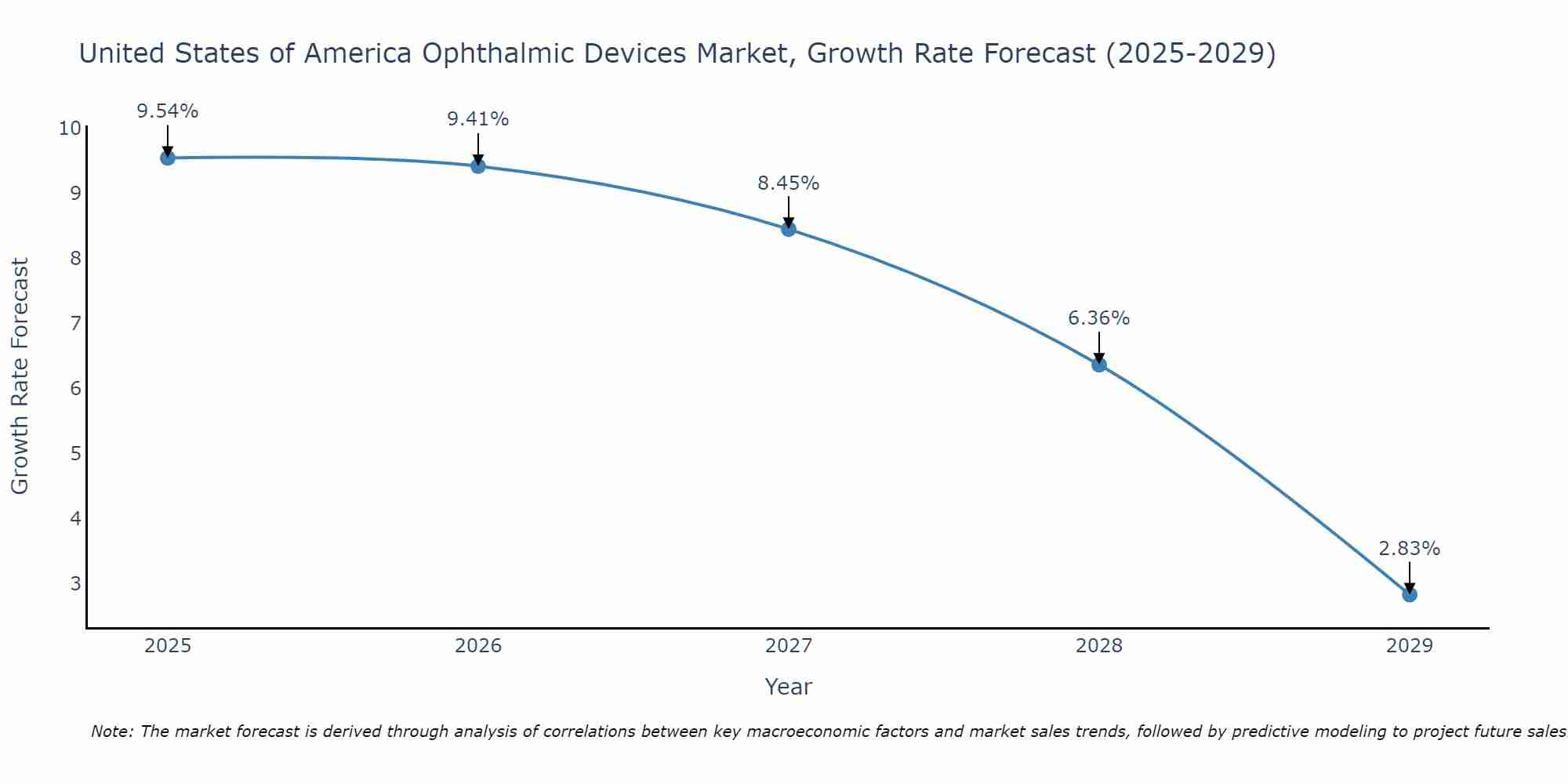
United States of America Ophthalmic Devices Market Size Growth Rate
The United States of America Ophthalmic Devices Market may undergo a gradual slowdown in growth rates between 2025 and 2029. Starting high at 9.54% in 2025, the market steadily declines to 2.83% by 2029.
United States (US) Ophthalmic Devices Market Synopsis
The United States Ophthalmic Devices Market is a rapidly growing healthcare sector driven by technological advancements, an aging population, and increasing prevalence of eye disorders such as cataracts, glaucoma, and age-related macular degeneration. The market encompasses a wide range of products including diagnostic devices, surgical instruments, vision care products, and intraocular lenses. Key market players include Alcon Inc., Johnson & Johnson Vision Care, Bausch + Lomb, and Carl Zeiss Meditec AG. With a strong focus on innovation and research, the US Ophthalmic Devices Market continues to witness steady growth, offering a variety of solutions to meet the diverse needs of ophthalmologists and patients alike. The market is expected to expand further with the adoption of advanced technologies like robotic-assisted surgery and artificial intelligence in eye care.
United States (US) Ophthalmic Devices Market Trends
The US Ophthalmic Devices Market is experiencing several key trends. Firstly, there is a growing demand for minimally invasive surgical procedures, driving the adoption of advanced technologies such as laser-assisted cataract surgery and implantable intraocular lenses. Secondly, the increasing prevalence of eye disorders and diseases, particularly among the aging population, is fueling the demand for diagnostic and monitoring devices such as optical coherence tomography and fundus cameras. Thirdly, the rise of telemedicine and remote monitoring solutions in ophthalmology is facilitating better access to eye care services, especially in rural areas. Lastly, there is a focus on developing innovative contact lenses and vision correction products to address the evolving needs of consumers, including those seeking options for myopia control and digital eye strain relief.
United States (US) Ophthalmic Devices Market Challenges
The US Ophthalmic Devices Market faces several challenges, including increasing competition from international players, stringent regulatory requirements, and rising healthcare costs. International competitors often offer lower-priced alternatives, putting pressure on domestic manufacturers to innovate and maintain competitive pricing. Additionally, regulatory hurdles can delay product approvals and increase time-to-market, impacting companies` ability to introduce new technologies promptly. Furthermore, the increasing cost of healthcare in the US poses a challenge for consumers and healthcare providers, leading to a more cautious approach towards adopting new ophthalmic devices. Navigating these challenges requires companies in the US Ophthalmic Devices Market to focus on innovation, compliance, cost efficiency, and strategic pricing strategies to stay competitive and meet the evolving needs of the market.
United States (US) Ophthalmic Devices Market Investment Opportunities
The US Ophthalmic Devices Market offers attractive investment opportunities due to factors such as the aging population, increasing prevalence of eye diseases, technological advancements, and growing demand for minimally invasive surgeries. Investors can consider opportunities in areas such as diagnostic devices (e.g., optical coherence tomography, fundus cameras), surgical devices (e.g., phacoemulsification systems, femtosecond lasers), and vision care products (e.g., contact lenses, spectacle lenses). Key players in the market include Alcon Inc., Johnson & Johnson Vision Care, Bausch + Lomb, and Carl Zeiss Meditec AG. Investing in innovative technologies and products that cater to the evolving needs of ophthalmic care providers and patients can potentially yield significant returns in this growing market.
Jordan Agar Market Government Policies
The United States government regulates the ophthalmic devices market through the Food and Drug Administration (FDA), which ensures product safety and efficacy. The FDA requires manufacturers to obtain premarket approval for certain ophthalmic devices, such as intraocular lenses and diagnostic devices. Additionally, the government has implemented reimbursement policies through Medicare and Medicaid, which impact the pricing and utilization of ophthalmic devices. The Affordable Care Act also influences the market by expanding insurance coverage for vision care services, potentially increasing demand for ophthalmic devices. Overall, government policies in the US ophthalmic devices market focus on ensuring patient safety, promoting innovation, and expanding access to vision care services.
United States (US) Ophthalmic Devices Market Future Outlook
The United States Ophthalmic Devices Market is expected to experience steady growth in the coming years, driven by factors such as an aging population, increasing prevalence of eye diseases, technological advancements in the field, and rising awareness about eye health. The market is likely to see continued innovation in diagnostic and surgical devices, as well as a growing demand for contact lenses, intraocular lenses, and other vision correction products. Additionally, the increasing adoption of minimally invasive procedures and the shift towards value-based healthcare are expected to further fuel market growth. However, challenges such as regulatory hurdles, pricing pressures, and competition from alternative therapies may impact market dynamics. Overall, the US Ophthalmic Devices Market is poised for expansion as the demand for advanced eye care solutions continues to rise.
Key Highlights of the Report:
- United States (US) Ophthalmic Devices Market Outlook
- Market Size of United States (US) Ophthalmic Devices Market, 2021
- Forecast of United States (US) Ophthalmic Devices Market, 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Revenues & Volume for the Period 2018 - 2031
- United States (US) Ophthalmic Devices Market Trend Evolution
- United States (US) Ophthalmic Devices Market Drivers and Challenges
- United States (US) Ophthalmic Devices Price Trends
- United States (US) Ophthalmic Devices Porter's Five Forces
- United States (US) Ophthalmic Devices Industry Life Cycle
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Product for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Optical Coherence Tomography Scanners for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Fundus Cameras for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Perimeters/Visual Field Analyzers for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Autorefractors and Keratometers for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Ophthalmic Ultrasound Imaging Systems for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Ophthalmic Pachymeters for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Tonometers for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Optical Coherence Tomography Scanners Ophthalmic Devices Market Revenues & Volume By Slit Lamps for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Application for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Cataract for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Vitreo retinal disorders for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Glaucoma for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Refractor Disorders for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By End-use for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Hospitals and Eye Clinics for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Academic and Research Laboratory for the Period 2018 - 2031
- Historical Data and Forecast of United States (US) Ophthalmic Devices Market Revenues & Volume By Others for the Period 2018 - 2031
- United States (US) Ophthalmic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End-use
- United States (US) Ophthalmic Devices Top Companies Market Share
- United States (US) Ophthalmic Devices Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Ophthalmic Devices Company Profiles
- United States (US) Ophthalmic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Uganda Precast and Aggregate Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Australia IT Asset Disposal Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













