United States (US) Orthopedic Prosthetic Devices Market (2025-2031) Outlook | Value, Industry, Growth, Revenue, Share, Size, Analysis, Forecast, Trends & Companies
| Product Code: ETC275401 | Publication Date: Aug 2022 | Updated Date: Nov 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Dhaval Chaurasia | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
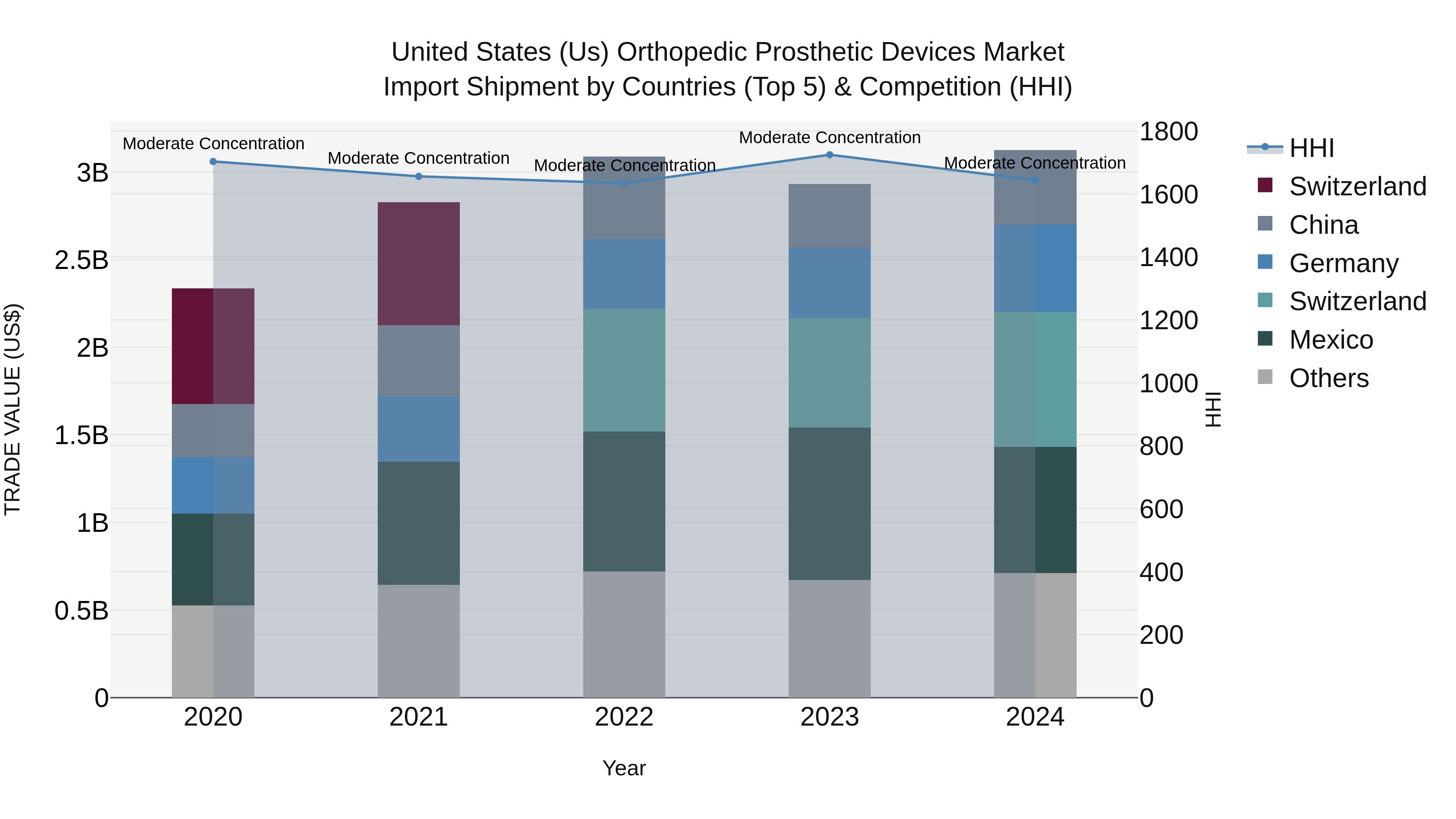
United States (US) Orthopedic Prosthetic Devices Market Top 5 Importing Countries and Market Competition (HHI) Analysis
In 2024, the United States continued to see steady growth in orthopedic prosthetic devices imports, with key exporting countries being Switzerland, Mexico, Germany, China, and Costa Rica. The market concentration, as measured by the HHI, remained moderate, indicating a healthy level of competition. The compound annual growth rate (CAGR) from 2020 to 2024 stood at 7.54%, reflecting sustained demand for orthopedic prosthetic devices. Moreover, the growth rate from 2023 to 2024 was reported at 6.57%, underscoring the sector`s resilience and potential for further expansion in the coming years.
United States (US) Orthopedic Prosthetic Devices Market Overview
The US Orthopedic Prosthetic Devices Market is a rapidly growing sector within the medical devices industry, driven by an aging population, increasing prevalence of chronic conditions such as osteoarthritis and osteoporosis, and advancements in technology. The market encompasses a wide range of products, including prosthetic limbs, joint implants, and orthopedic braces and supports. Key players in the market include established companies like Stryker Corporation, Zimmer Biomet Holdings, and Smith & Nephew plc, as well as smaller innovative firms focusing on developing cutting-edge prosthetic solutions. With a strong emphasis on research and development, the US orthopedic prosthetic devices market is poised for continued growth, with a focus on improving patient outcomes, enhancing mobility, and increasing overall quality of life for individuals with orthopedic impairments.
United States (US) Orthopedic Prosthetic Devices Market Trends
The US Orthopedic Prosthetic Devices Market is witnessing several key trends. One significant trend is the increasing adoption of advanced technologies such as 3D printing and robotics in the manufacturing of orthopedic prosthetic devices, leading to more personalized and precise solutions for patients. Another trend is the growing demand for prosthetic devices among an aging population and individuals with musculoskeletal disorders or injuries, driving market growth. Additionally, there is a rising focus on developing prosthetic devices that offer improved functionality, comfort, and durability to enhance patients` quality of life. Moreover, advancements in materials science and design techniques are enabling the development of lighter and more aesthetically pleasing prosthetic devices. Overall, these trends are shaping the US Orthopedic Prosthetic Devices Market towards innovation, customization, and improved patient outcomes.
United States (US) Orthopedic Prosthetic Devices Market Challenges
The US Orthopedic Prosthetic Devices Market faces several challenges, including stringent regulatory requirements, increasing healthcare costs, and competition from non-traditional players. Regulatory approvals for new prosthetic devices can be time-consuming and costly, leading to delays in market entry. Rising healthcare costs and reimbursement challenges also impact the market, making it difficult for patients to afford expensive prosthetic devices. Additionally, the market is witnessing competition from innovative technologies and materials, as well as from new entrants such as 3D printing companies and startups. Adapting to these challenges by focusing on innovation, cost-efficiency, and regulatory compliance will be crucial for companies operating in the US Orthopedic Prosthetic Devices Market to maintain growth and competitive advantage.
United States (US) Orthopedic Prosthetic Devices Market Investment Opportunities
The United States Orthopedic Prosthetic Devices Market offers promising investment opportunities due to factors such as a growing aging population, increasing prevalence of orthopedic conditions, advancements in technology leading to innovative prosthetic devices, and rising demand for personalized and customized prosthetic solutions. Investors can benefit from investing in companies that specialize in developing cutting-edge prosthetic devices, such as implantable prosthetics, wearable robotic exoskeletons, and 3D-printed custom prostheses. Additionally, investing in companies that focus on research and development to enhance the functionality and durability of orthopedic prosthetic devices can be lucrative in this market. Overall, the US orthopedic prosthetic devices market presents a favorable landscape for investment opportunities driven by evolving consumer needs and technological advancements.
United States (US) Orthopedic Prosthetic Devices Market Government Policy
The US government regulates the Orthopedic Prosthetic Devices Market through various policies and regulations enforced by the Food and Drug Administration (FDA). These regulations ensure the safety, effectiveness, and quality of orthopedic prosthetic devices sold in the market. Manufacturers are required to obtain FDA approval or clearance before marketing their products, and they must adhere to stringent quality control processes and reporting requirements. Additionally, government healthcare programs such as Medicare and Medicaid play a significant role in reimbursement policies for orthopedic prosthetic devices, which impacts market access and pricing. Overall, government policies in the US Orthopedic Prosthetic Devices Market aim to protect consumer safety, promote innovation, and ensure access to necessary medical devices for patients in need.
United States (US) Orthopedic Prosthetic Devices Market Future Outlook
The future outlook for the United States Orthopedic Prosthetic Devices Market appears promising, driven by factors such as an aging population, increasing prevalence of musculoskeletal disorders, technological advancements in prosthetic devices, and rising awareness about the benefits of orthopedic prosthetics. The market is expected to witness steady growth as innovations in materials and design lead to more personalized and efficient prosthetic solutions. Additionally, the growing adoption of advanced surgical techniques and robotics in orthopedic procedures is anticipated to further boost market growth. However, challenges such as stringent regulatory requirements and high costs associated with prosthetic devices may hinder the market expansion to some extent. Overall, the US Orthopedic Prosthetic Devices Market is poised for continued development and innovation in the coming years.
Key Highlights of the Report:
- United States (US) Orthopedic Prosthetic Devices Market Outlook
- Market Size of United States (US) Orthopedic Prosthetic Devices Market, 2024
- Forecast of United States (US) Orthopedic Prosthetic Devices Market, 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Revenues & Volume for the Period 2021 - 2031
- United States (US) Orthopedic Prosthetic Devices Market Trend Evolution
- United States (US) Orthopedic Prosthetic Devices Market Drivers and Challenges
- United States (US) Orthopedic Prosthetic Devices Price Trends
- United States (US) Orthopedic Prosthetic Devices Porter's Five Forces
- United States (US) Orthopedic Prosthetic Devices Industry Life Cycle
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Product Type for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Upper Extremity Prosthetics for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Lower Extremity Prosthetics for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Liners for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Sockets for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Modular Components for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Technology for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Conventional for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Electric Powered for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Hybrid Orthopedic Prosthetics for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By End User for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Hospitals for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Prosthetic Clinics for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Rehabilitation Center for the Period 2021 - 2031
- Historical Data and Forecast of United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume By Others for the Period 2021 - 2031
- United States (US) Orthopedic Prosthetic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product Type
- Market Opportunity Assessment By Technology
- Market Opportunity Assessment By End User
- United States (US) Orthopedic Prosthetic Devices Top Companies Market Share
- United States (US) Orthopedic Prosthetic Devices Competitive Benchmarking By Technical and Operational Parameters
- United States (US) Orthopedic Prosthetic Devices Company Profiles
- United States (US) Orthopedic Prosthetic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
1 Executive Summary |
2 Introduction |
2.1 Key Highlights of the Report |
2.2 Report Description |
2.3 Market Scope & Segmentation |
2.4 Research Methodology |
2.5 Assumptions |
3 United States (US) Orthopedic Prosthetic Devices Market Overview |
3.1 United States (US) Country Macro Economic Indicators |
3.2 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, 2021 & 2031F |
3.3 United States (US) Orthopedic Prosthetic Devices Market - Industry Life Cycle |
3.4 United States (US) Orthopedic Prosthetic Devices Market - Porter's Five Forces |
3.5 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume Share, By Product Type, 2021 & 2031F |
3.6 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume Share, By Technology, 2021 & 2031F |
3.7 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume Share, By End User, 2021 & 2031F |
4 United States (US) Orthopedic Prosthetic Devices Market Dynamics |
4.1 Impact Analysis |
4.2 Market Drivers |
4.2.1 Increasing prevalence of orthopedic disorders and injuries leading to higher demand for orthopedic prosthetic devices |
4.2.2 Technological advancements in prosthetic devices leading to improved functionality and comfort for patients |
4.2.3 Growing aging population in the United States driving the need for orthopedic prosthetic devices |
4.3 Market Restraints |
4.3.1 High cost associated with orthopedic prosthetic devices limiting accessibility for some patients |
4.3.2 Stringent regulatory requirements for approval and manufacturing of prosthetic devices impacting market growth |
4.3.3 Limited reimbursement policies for orthopedic prosthetic devices affecting adoption rates |
5 United States (US) Orthopedic Prosthetic Devices Market Trends |
6 United States (US) Orthopedic Prosthetic Devices Market, By Types |
6.1 United States (US) Orthopedic Prosthetic Devices Market, By Product Type |
6.1.1 Overview and Analysis |
6.1.2 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Product Type, 2021 - 2031F |
6.1.3 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Upper Extremity Prosthetics, 2021 - 2031F |
6.1.4 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Lower Extremity Prosthetics, 2021 - 2031F |
6.1.5 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Liners, 2021 - 2031F |
6.1.6 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Sockets, 2021 - 2031F |
6.1.7 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Modular Components, 2021 - 2031F |
6.2 United States (US) Orthopedic Prosthetic Devices Market, By Technology |
6.2.1 Overview and Analysis |
6.2.2 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Conventional, 2021 - 2031F |
6.2.3 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Electric Powered, 2021 - 2031F |
6.2.4 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Hybrid Orthopedic Prosthetics, 2021 - 2031F |
6.3 United States (US) Orthopedic Prosthetic Devices Market, By End User |
6.3.1 Overview and Analysis |
6.3.2 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Hospitals, 2021 - 2031F |
6.3.3 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Prosthetic Clinics, 2021 - 2031F |
6.3.4 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Rehabilitation Center, 2021 - 2031F |
6.3.5 United States (US) Orthopedic Prosthetic Devices Market Revenues & Volume, By Others, 2021 - 2031F |
7 United States (US) Orthopedic Prosthetic Devices Market Import-Export Trade Statistics |
7.1 United States (US) Orthopedic Prosthetic Devices Market Export to Major Countries |
7.2 United States (US) Orthopedic Prosthetic Devices Market Imports from Major Countries |
8 United States (US) Orthopedic Prosthetic Devices Market Key Performance Indicators |
8.1 Average waiting time for prosthetic device fittings |
8.2 Patient satisfaction rates with orthopedic prosthetic devices |
8.3 Number of successful prosthetic device implantations |
8.4 Rate of adoption of technologically advanced prosthetic devices |
8.5 Number of clinical trials and research studies on orthopedic prosthetic devices |
9 United States (US) Orthopedic Prosthetic Devices Market - Opportunity Assessment |
9.1 United States (US) Orthopedic Prosthetic Devices Market Opportunity Assessment, By Product Type, 2021 & 2031F |
9.2 United States (US) Orthopedic Prosthetic Devices Market Opportunity Assessment, By Technology, 2021 & 2031F |
9.3 United States (US) Orthopedic Prosthetic Devices Market Opportunity Assessment, By End User, 2021 & 2031F |
10 United States (US) Orthopedic Prosthetic Devices Market - Competitive Landscape |
10.1 United States (US) Orthopedic Prosthetic Devices Market Revenue Share, By Companies, 2024 |
10.2 United States (US) Orthopedic Prosthetic Devices Market Competitive Benchmarking, By Operating and Technical Parameters |
11 Company Profiles |
12 Recommendations |
13 Disclaimer |
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Thought Leadership and Analyst Meet
Our Clients
Related Reports
- Afghanistan Apparel Market (2026-2032) | Growth, Outlook, Industry, Segmentation, Forecast, Size, Companies, Trends, Value, Share, Analysis & Revenue
- Canada Oil and Gas Market (2026-2032) | Share, Segmentation, Value, Industry, Trends, Forecast, Analysis, Size & Revenue, Growth, Competitive Landscape, Outlook, Companies
- Germany Breakfast Food Market (2026-2032) | Industry, Share, Growth, Size, Companies, Value, Analysis, Revenue, Trends, Forecast & Outlook
- Australia Briquette Market (2025-2031) | Growth, Size, Revenue, Forecast, Analysis, Trends, Value, Share, Industry & Companies
- Vietnam System Integrator Market (2025-2031) | Size, Companies, Analysis, Industry, Value, Forecast, Growth, Trends, Revenue & Share
- ASEAN and Thailand Brain Health Supplements Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero


















