United Kingdom (UK) Ophthalmic Devices Market (2025-2031) Outlook | Value, Revenue, Industry, Growth, Analysis, Companies, Trends, Share, Forecast & Size
| Product Code: ETC367869 | Publication Date: Aug 2022 | Updated Date: Jul 2025 | Product Type: Market Research Report | |
| Publisher: 6Wresearch | Author: Ravi Bhandari | No. of Pages: 75 | No. of Figures: 35 | No. of Tables: 20 |
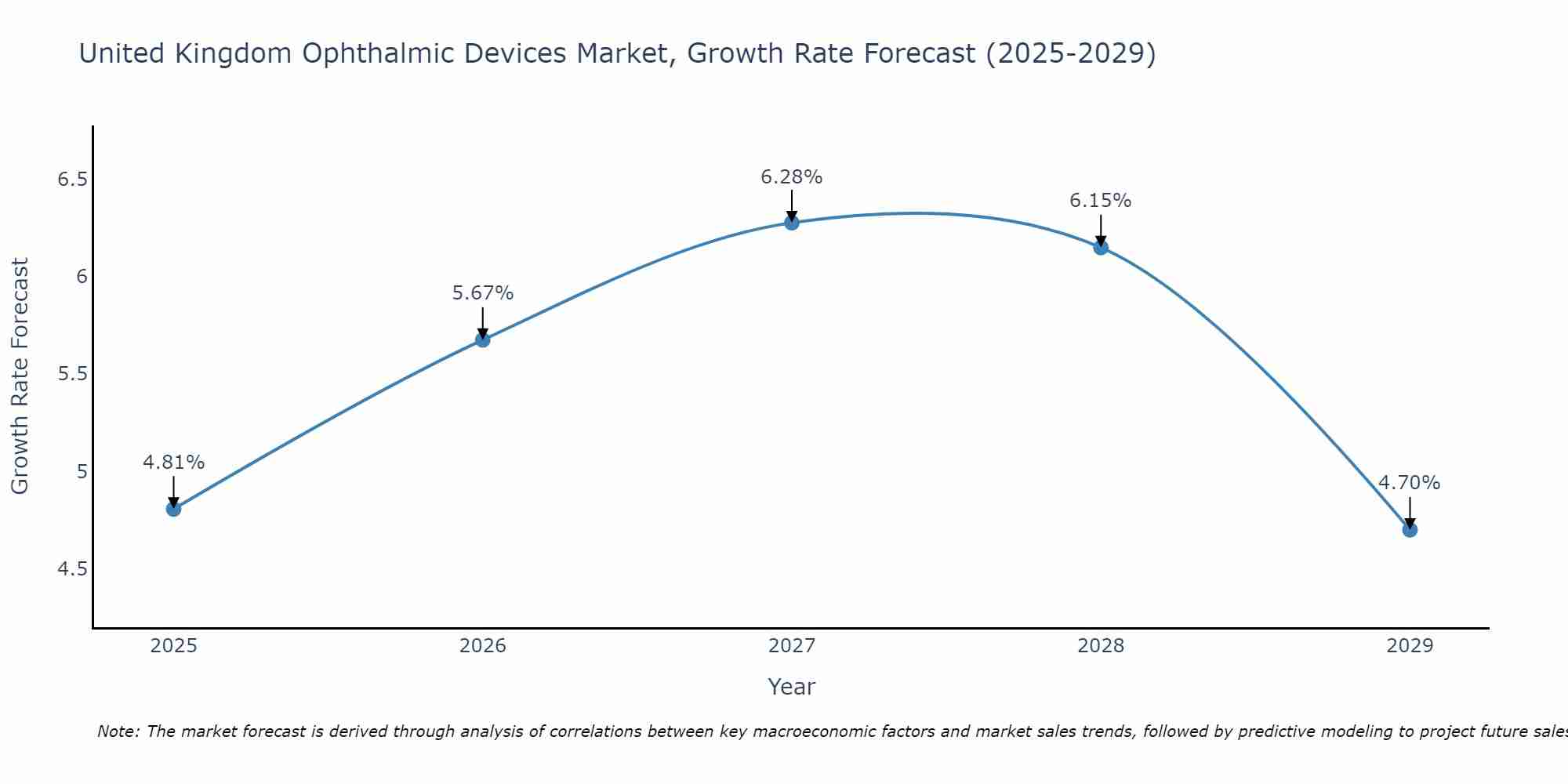
United Kingdom Ophthalmic Devices Market Size Growth Rate
The United Kingdom Ophthalmic Devices Market is projected to witness mixed growth rate patterns during 2025 to 2029. The growth rate begins at 4.81% in 2025, climbs to a high of 6.28% in 2027, and moderates to 4.70% by 2029.
United Kingdom (UK) Ophthalmic Devices Market Synopsis
The United Kingdom (UK) Ophthalmic Devices Market is a rapidly growing sector driven by advancements in technology and an aging population. The market encompasses a wide range of devices such as diagnostic equipment, surgical instruments, vision care products, and more. Key drivers of growth include an increasing prevalence of eye diseases such as cataracts, glaucoma, and age-related macular degeneration, leading to a higher demand for ophthalmic devices. Additionally, the rising adoption of minimally invasive surgical procedures and the increasing awareness about eye health are also contributing to market expansion. Major players in the UK ophthalmic devices market include Alcon, Bausch + Lomb, Carl Zeiss Meditec, and Johnson & Johnson Vision. Overall, the market is poised for continued growth as technological innovations drive the development of more effective and efficient ophthalmic devices.
United Kingdom (UK) Ophthalmic Devices Market Trends
The United Kingdom (UK) Ophthalmic Devices Market is experiencing several key trends currently. One prominent trend is the increasing adoption of advanced technologies such as robotics, artificial intelligence, and digital imaging in ophthalmic devices. These technologies are enhancing the accuracy and efficiency of diagnostics, surgeries, and treatments in the field of ophthalmology. Another trend is the rising prevalence of eye disorders and conditions, particularly among the aging population, driving the demand for ophthalmic devices for diagnosis and treatment. Additionally, there is a growing focus on developing innovative and minimally invasive ophthalmic devices to improve patient outcomes and reduce recovery times. Overall, the UK Ophthalmic Devices Market is witnessing a shift towards cutting-edge technologies and patient-centric solutions to address the evolving needs of healthcare providers and patients.
United Kingdom (UK) Ophthalmic Devices Market Challenges
In the UK Ophthalmic Devices Market, challenges are primarily driven by factors such as changing regulatory requirements, increasing competition, and technological advancements. The market faces challenges related to ensuring compliance with strict regulations governing the development and marketing of ophthalmic devices, which can impact product innovation and time-to-market. Additionally, the competitive landscape is intensifying with the entry of new players offering innovative solutions, putting pressure on established companies to differentiate themselves. Keeping pace with rapid technological advancements and incorporating them into product offerings while ensuring affordability for patients is another challenge faced by players in the UK Ophthalmic Devices Market. Overall, navigating these challenges requires companies to prioritize regulatory compliance, invest in research and development, and adopt strategic pricing and marketing strategies to stay competitive in the market.
United Kingdom (UK) Ophthalmic Devices Market Investment Opportunities
The United Kingdom (UK) ophthalmic devices market presents a promising investment opportunity due to several factors. The growing prevalence of eye disorders and diseases such as cataracts, age-related macular degeneration, and glaucoma drives the demand for advanced ophthalmic devices and treatments. Technological advancements in the field, such as the development of innovative diagnostic tools, surgical equipment, and artificial intelligence-driven solutions, further contribute to market growth. Additionally, the aging population in the UK increases the need for eye care services, creating a sustained market demand. Investing in the UK ophthalmic devices market offers potential for significant returns, particularly in companies that focus on cutting-edge technologies, personalized treatments, and addressing unmet needs in the eye care sector.
Jordan Agar Market Government Policies
The UK government closely regulates the ophthalmic devices market to ensure patient safety and quality standards. The Medicines and Healthcare products Regulatory Agency (MHRA) oversees the approval and monitoring of ophthalmic devices, ensuring they meet stringent regulatory requirements before entering the market. Additionally, the National Institute for Health and Care Excellence (NICE) provides guidance on the use of ophthalmic devices within the National Health Service (NHS), influencing procurement decisions. The government also promotes innovation in the sector through funding opportunities and research grants to drive advancements in ophthalmic technology. Overall, government policies in the UK ophthalmic devices market prioritize patient safety, quality assurance, and accessibility to innovative technologies.
United Kingdom (UK) Ophthalmic Devices Market Future Outlook
The future outlook for the United Kingdom (UK) Ophthalmic Devices Market appears promising, driven by factors such as an aging population, increasing prevalence of eye disorders, technological advancements in ophthalmic devices, and growing awareness about eye health. The market is expected to witness steady growth as demand for diagnostic and surgical ophthalmic devices rises. Additionally, the adoption of advanced treatments like laser eye surgery and minimally invasive procedures is expected to further boost market growth. However, challenges such as pricing pressures, regulatory hurdles, and competition among key players may impact the market dynamics. Overall, the UK Ophthalmic Devices Market is poised for expansion in the coming years, offering opportunities for innovation and market growth.
Key Highlights of the Report:
- United Kingdom (UK) Ophthalmic Devices Market Outlook
- Market Size of United Kingdom (UK) Ophthalmic Devices Market, 2024
- Forecast of United Kingdom (UK) Ophthalmic Devices Market, 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Revenues & Volume for the Period 2021 - 2031
- United Kingdom (UK) Ophthalmic Devices Market Trend Evolution
- United Kingdom (UK) Ophthalmic Devices Market Drivers and Challenges
- United Kingdom (UK) Ophthalmic Devices Price Trends
- United Kingdom (UK) Ophthalmic Devices Porter's Five Forces
- United Kingdom (UK) Ophthalmic Devices Industry Life Cycle
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Product for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Optical Coherence Tomography Scanners for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Fundus Cameras for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Perimeters/Visual Field Analyzers for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Autorefractors and Keratometers for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Ophthalmic Ultrasound Imaging Systems for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Ophthalmic Pachymeters for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Tonometers for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Optical Coherence Tomography Scanners Ophthalmic Devices Market Revenues & Volume By Slit Lamps for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Application for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Cataract for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Vitreo retinal disorders for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Glaucoma for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Refractor Disorders for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By End-use for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Hospitals and Eye Clinics for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Academic and Research Laboratory for the Period 2021 - 2031
- Historical Data and Forecast of United Kingdom (UK) Ophthalmic Devices Market Revenues & Volume By Others for the Period 2021 - 2031
- United Kingdom (UK) Ophthalmic Devices Import Export Trade Statistics
- Market Opportunity Assessment By Product
- Market Opportunity Assessment By Application
- Market Opportunity Assessment By End-use
- United Kingdom (UK) Ophthalmic Devices Top Companies Market Share
- United Kingdom (UK) Ophthalmic Devices Competitive Benchmarking By Technical and Operational Parameters
- United Kingdom (UK) Ophthalmic Devices Company Profiles
- United Kingdom (UK) Ophthalmic Devices Key Strategic Recommendations
Frequently Asked Questions About the Market Study (FAQs):
- Single User License$ 1,995
- Department License$ 2,400
- Site License$ 3,120
- Global License$ 3,795
Search
Related Reports
- ASEAN Bearings Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Europe Flooring Market (2025-2031) | Outlook, Share, Industry, Trends, Forecast, Companies, Revenue, Size, Analysis, Growth & Value
- Saudi Arabia Manlift Market (2025-2031) | Outlook, Size, Growth, Trends, Companies, Industry, Revenue, Value, Share, Forecast & Analysis
- Uganda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Rwanda Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Kenya Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Angola Excavator, Crane, and Wheel Loaders Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Israel Intelligent Transport System Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Uganda Precast and Aggregate Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
- Australia IT Asset Disposal Market (2025-2031) | Strategy, Consumer Insights, Analysis, Investment Trends, Opportunities, Growth, Size, Share, Industry, Revenue, Segments, Value, Segmentation, Supply, Forecast, Restraints, Outlook, Competition, Drivers, Trends, Demand, Pricing Analysis, Competitive, Strategic Insights, Companies, Challenges
Industry Events and Analyst Meet
Our Clients
Whitepaper
- Middle East & Africa Commercial Security Market Click here to view more.
- Middle East & Africa Fire Safety Systems & Equipment Market Click here to view more.
- GCC Drone Market Click here to view more.
- Middle East Lighting Fixture Market Click here to view more.
- GCC Physical & Perimeter Security Market Click here to view more.
6WResearch In News
- Doha a strategic location for EV manufacturing hub: IPA Qatar
- Demand for luxury TVs surging in the GCC, says Samsung
- Empowering Growth: The Thriving Journey of Bangladesh’s Cable Industry
- Demand for luxury TVs surging in the GCC, says Samsung
- Video call with a traditional healer? Once unthinkable, it’s now common in South Africa
- Intelligent Buildings To Smooth GCC’s Path To Net Zero













